Learn more about the Renal System
In this module, you will learn more about
- Kidney anatomy
- The kidney nephron
- Nephron function: filtration, reabsorption and secretion
- Transport, storage and excretion of urine
Learn even more: See Chemistry and Cells modules
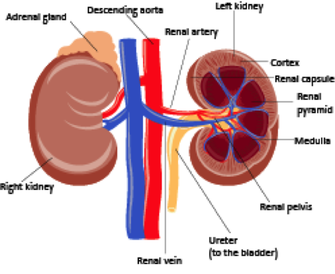
1 Kidney anatomy
The kidneys lie retro-peritoneally in the superior lumbar region; the right kidney lies slightly lower than the left.
They are bean-shaped organs, weighing approximately 150 g, are about 11 cm long by 6 cm wide, and 3 cm thick.
Renal blood vessels, lymph vessels, nerves and ureters all join the kidney at the hilum on the medial surface of the kidney.
An adrenal gland sits on the superior surface of each kidney and is functionally unrelated to the kidneys.
The kidney is divided into the cortex (outer region) the medulla and the renal pelvis which is continuous with the ureters. Normally one ureter leaves each kidney and connects to the urinary bladder.
They are bean-shaped organs, weighing approximately 150 g, are about 11 cm long by 6 cm wide, and 3 cm thick.
Renal blood vessels, lymph vessels, nerves and ureters all join the kidney at the hilum on the medial surface of the kidney.
An adrenal gland sits on the superior surface of each kidney and is functionally unrelated to the kidneys.
The kidney is divided into the cortex (outer region) the medulla and the renal pelvis which is continuous with the ureters. Normally one ureter leaves each kidney and connects to the urinary bladder.
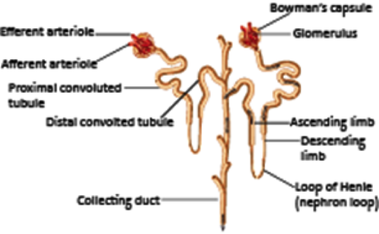
2 The kidney nephron
The structural and functional unit of the kidney is the nephron. There are over one million in each kidney, of which 85 % are cortical nephrons, and 15 % are juxtamedullary nephrons that span both the cortex and medulla.
The nephron is composed of a filtration membrane, the glomerulus, a series of tubules and a collecting duct.
Cortical nephrons are located in the kidney cortex with their short loop of Henle loop crossing into the medulla, while juxtaglomerular nephrons are located at the cortical-medullary interface and have long loops of Henle that run deep into the medulla.
The nephron is composed of a filtration membrane, the glomerulus, a series of tubules and a collecting duct.
Cortical nephrons are located in the kidney cortex with their short loop of Henle loop crossing into the medulla, while juxtaglomerular nephrons are located at the cortical-medullary interface and have long loops of Henle that run deep into the medulla.
About 1200 ml blood enters the kidneys via the renal arteries each minute. These arteries divide into smaller vessels until they reach the afferent arterioles where blood enters the glomeruli. Blood then exits the glomerulus via an efferent arteriole. As blood moves through the glomerulus substances are filtered out of the blood and into the Bowman’s capsule. This filtrate then travels along the proximal convoluted tubule (PCT), into the nephron loop, though the distal convoluted tubule (DCT), into one of the thousands of collecting ducts. The filtrate then enters the renal pelvis and flows into the ureter to the bladder.
Surrounding the tubules of the cortical nephrons are peritubular capillaries which are continuous with the efferent nephron, and are the site of reabsorption. The nephron loops of the juxtaglomerular nephrons are surrounded by adjacent parallel capillaries, known as the vasa recta. Blood flows in the opposite direction to the flow of the tubular fluid, and, along with the long loops of Henle, are responsible for creating a medullary gradient which is important for urine formation.
Along the tubules substances, such as electrolytes and water, are reabsorbed and secreted. These three processes; glomerular filtration, tubular reabsorption and tubular secretion, all help to maintain blood homeostasis by removing unwanted substances from the blood and excreting them as urine.
Surrounding the tubules of the cortical nephrons are peritubular capillaries which are continuous with the efferent nephron, and are the site of reabsorption. The nephron loops of the juxtaglomerular nephrons are surrounded by adjacent parallel capillaries, known as the vasa recta. Blood flows in the opposite direction to the flow of the tubular fluid, and, along with the long loops of Henle, are responsible for creating a medullary gradient which is important for urine formation.
Along the tubules substances, such as electrolytes and water, are reabsorbed and secreted. These three processes; glomerular filtration, tubular reabsorption and tubular secretion, all help to maintain blood homeostasis by removing unwanted substances from the blood and excreting them as urine.
3 Nephron function: filtration, reabsorption and secretion
Let’s look at the individual parts of the nephron and the role they play in urine formation.
Glomerular Filtration
The endothelial cells of the glomerular capillaries are fenestrated which allows fluid, and substances smaller than 5 nm to be filtered out of the blood and enter the capsular space. Glomerular filtration is a passive process by which the hydrostatic pressure of the blood entering the glomerulus (filtration pressure), forces solutes and fluids out of the blood in to the capsule. Plasma proteins (albumin, globulins and fibrinogen) do not cross the filtration membrane because they are too big. Urine therefore does not normally contain any proteins. The plasma proteins maintain colloid osmotic pressure of he blood to prevent loss of all water into the Bowman’s capsule.
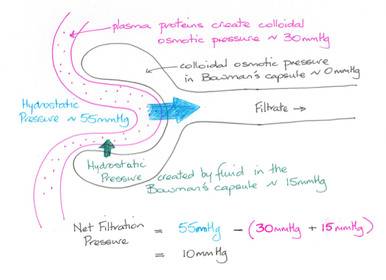
Net filtration pressure
As with capillary beds elsewhere hydrostatic pressure and colloid osmotic pressure control the flow of substances across the glomerular capillaries and into the capsular space; the net filtration pressure (NFR). Within the glomerular capillaries hydrostatic pressure (the blood pressure), is about 55 mm Hg, and pushes substances out of the capillaries, while colloid osmotic pressure is about 30 mm Hg. Within the capsular space colloid osmotic pressure is essentially zero, and hydrostatic pressure is about 15 mm Hg.
The NFR is equal to the outward pressures (into the capsular space) less the inwards pressures (into the blood).
(55 + 0) – (15 + 30) = 10 mm Hg, and determines the glomerular filtration rate (GFR).
The NFR is equal to the outward pressures (into the capsular space) less the inwards pressures (into the blood).
(55 + 0) – (15 + 30) = 10 mm Hg, and determines the glomerular filtration rate (GFR).
Glomerular filtration rate
The glomerular filtration rate (GFR) is the amount of filtrate formed each minute by all the glomeruli in both kidneys and is about 125 ml/min. It is proportional to the net filtration pressure (NFP), the surface area available for filtration and the permeability of the filtration membrane. By far the most significant is the NFP which can be controlled by altering the diameter of the afferent and sometimes the efferent arterioles. Both intrinsic and extrinsic mechanisms control GFR and these are discussed briefly.
Intrinsic controls act within the kidney and are known as renal autoregulation. They maintain GFR on a daily basis over a BP range of 80 – 180 mm Hg, which encompasses the changes that take place during exercise, sleeping, and other day-to-day activities. There are two mechanisms:
Extrinsic controls are neural and hormonal mechanisms which respond to extremely low BP (BP < 80 mmHg).
Intrinsic controls act within the kidney and are known as renal autoregulation. They maintain GFR on a daily basis over a BP range of 80 – 180 mm Hg, which encompasses the changes that take place during exercise, sleeping, and other day-to-day activities. There are two mechanisms:
- The myogenic mechanism relies on properties of vascular smooth muscle. If systemic blood pressure (BP), rises vascular smooth muscle in the arteriolar walls responds by constricting, decreasing the amount of blood entering the glomerulus and prevents a rise in glomerular blood pressure. Conversely, if BP falls then the afferent arterioles dilate, increasing blood flow to the glomeruli and maintaining NFP and GFR.
- The tubuloglomerular feedback mechanism relies on a group of cells situated in the ascending limb of the nephron loop. These macula denser cells respond to the concentration of sodium chloride (NaCl), in the filtrate. When GFR increases, NaCl rise in the filtrate and the macula denser cells respond by releasing vasoconstrictor chemicals which cause the afferent arterioles to constrict thus reducing blood flow and thus NFP and GFR. Low NaCl levels in the filtrate levels leads to vasodilation, increased blood flow and an increase in NFP and GFR.
Extrinsic controls are neural and hormonal mechanisms which respond to extremely low BP (BP < 80 mmHg).
- Neural controls override autoregulatory mechanisms when blood volume becomes extremely low, as in hypovolemic shock. Sympathetic nerve fibres release noradrenalin (norepinephrine) causing peripheral vascular smooth muscle to constrict. This also causes the afferent arterioles to constrict reducing GFR, and helps increase blood volume and restore normal BP.
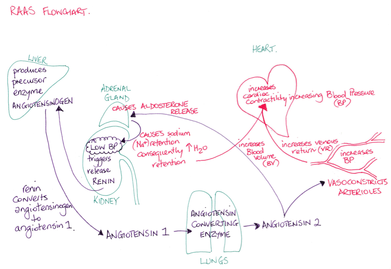
- The renin-angiotensin-aldosterone mechanism is the major mechanism by which low BP can be corrected. Juxtaglomerular cells (granular cells) in the juxtaglomerular complex respond to low BP by releasing renin. Renin triggers angiotensin I conversion to angiotensin II. Angiotensin II is a potent vasoconstrictor and it triggers aldosterone release by the adrenal glands. Aldosterone acts on the DCT and collecting ducts leading to increased NaCl reabsorption. Water follows sodium and thus blood volume increases contributing to an increase in BP.
Tubular reabsorption
Tubular reabsorption is the process of returning fluid and solutes to the blood once they enter the kidney tubules. Reabsorption can be active (requiring ATP) or passive by the mechanisms of diffusion, facilitated diffusion and osmosis. While most organic nutrients are completely reabsorbed, the reabsorption of water and electrolytes is tightly regulated and is influenced by hormones.
Reabsorption of the glomerular filtrate is most active in the PCT. Normally all the glucose and amino acids in the filtrate and 65 % of sodium are reabsorbed, along with nearly all the uric acid and 50 % of the urea found in the filtrate.
Within the Loop of Henle water reabsorption is not coupled with electrolyte reabsorption. The descending limb is permeable to water and not solutes, while in the ascending limb solutes are reabsorbed, but the cells are impermeable to water. This process is important for the kidneys to be able to either dilute or concentrate urine.
Reabsorption of the glomerular filtrate is most active in the PCT. Normally all the glucose and amino acids in the filtrate and 65 % of sodium are reabsorbed, along with nearly all the uric acid and 50 % of the urea found in the filtrate.
Within the Loop of Henle water reabsorption is not coupled with electrolyte reabsorption. The descending limb is permeable to water and not solutes, while in the ascending limb solutes are reabsorbed, but the cells are impermeable to water. This process is important for the kidneys to be able to either dilute or concentrate urine.
Hormone control of solute and water resorbption
Hormones fine-tune the amount of solute and water reabsorption within the DCT and CD.
ADH inhibits diuresis (water loss) by making the cells of the collecting duct more permeable to water by inserting aquaporins into their membranes. The number of aquaporins is determined by the hydration state of the body. When one is dehydrated the osmolarity of the extracellular fluid increases leading to the posterior pituitary gland increasing the secretion of ADH, resulting in more aquaporins so that more water is reabsorbed and less, more concentrated urine is produced.
Aldosterone acts on the distal part of the DCT and the CD in response to low BP, low blood volume, or increased extracellular potassium. Aldosterone release results in little or no sodium being excreted in the urine.
Atrial natriuretic peptide (ANP) is released by cardiac atrial cells when blood volume or blood pressure is elevated. This hormone inhibits sodium reabsorption in the CDs, leading to excretion of sodium and water causing blood volume to be reduced and as a consequence reduces BP.
ADH inhibits diuresis (water loss) by making the cells of the collecting duct more permeable to water by inserting aquaporins into their membranes. The number of aquaporins is determined by the hydration state of the body. When one is dehydrated the osmolarity of the extracellular fluid increases leading to the posterior pituitary gland increasing the secretion of ADH, resulting in more aquaporins so that more water is reabsorbed and less, more concentrated urine is produced.
Aldosterone acts on the distal part of the DCT and the CD in response to low BP, low blood volume, or increased extracellular potassium. Aldosterone release results in little or no sodium being excreted in the urine.
Atrial natriuretic peptide (ANP) is released by cardiac atrial cells when blood volume or blood pressure is elevated. This hormone inhibits sodium reabsorption in the CDs, leading to excretion of sodium and water causing blood volume to be reduced and as a consequence reduces BP.
Tubular secretion
Tubular secretion is the process of moving unwanted substances out of the peritubular capillaries into the filtrate to be excreted in urine. This process is important for removing certain drugs and metabolites, removing nitrogenous wastes, getting rid of excess potassium, and controlling blood pH by selectively secreting hydrogen ions.
On leaving the CDs the urine is collected in the calyces of the kidney from where it drains into the renal pelvis and on into the ureter.
The Ureters
The ureter is a narrow tube which is continuous with the kidney pelvis, and runs from the kidney to the bladder.
Ureters consist of three layers:
As urine enters the ureter it distends and stimulates the muscularis to contract to propel urine towards the bladder. The strength and frequency of these peristaltic waves is dependent on the rate of urine formation. Gravity also helps fluid move towards the bladder.
Ureters consist of three layers:
- the mucosa
- the muscularis
- the adventitia
As urine enters the ureter it distends and stimulates the muscularis to contract to propel urine towards the bladder. The strength and frequency of these peristaltic waves is dependent on the rate of urine formation. Gravity also helps fluid move towards the bladder.
The bladder
The bladder lies retroperitoneally on the pelvic floor, and has three layers; the mucosa, a thick muscular layer, called the detrusor muscle, and a fibrous adventitia.
The interior of the bladder has openings for the ureters and the urethra, which from a triangular region, known as the trigone, on the base of the bladder.
The bladder is distensible, collapsing when empty and expanding as urine enters via the ureters, expanding up to 1 L.
The interior of the bladder has openings for the ureters and the urethra, which from a triangular region, known as the trigone, on the base of the bladder.
The bladder is distensible, collapsing when empty and expanding as urine enters via the ureters, expanding up to 1 L.
The urethra
The urethra is a thin walled, muscular tube which carries urine away from the bladder to the outside world.
In females it is a short tube 3-4 cm long, while in males it is approximately 20 cm in length, and has a dual function; to carry urine and to carry sperm. The shorter length of the female urethra accounts for women being more susceptible to urinary tract infections, while in males the prostate gland circumscribes the superior aspect of the urethra (the prostatic urethra), and can cause constriction of the tube if the prostate gland becomes enlarged.
The detrusor muscle is thicker around the bladder-urethral junction, forming the involuntary internal urethral sphincter. This sphincter remains closed unless one is voiding. At the distal end of the urethra is the external urethral sphincter made up of skeletal muscle which is under voluntary control.
In females it is a short tube 3-4 cm long, while in males it is approximately 20 cm in length, and has a dual function; to carry urine and to carry sperm. The shorter length of the female urethra accounts for women being more susceptible to urinary tract infections, while in males the prostate gland circumscribes the superior aspect of the urethra (the prostatic urethra), and can cause constriction of the tube if the prostate gland becomes enlarged.
The detrusor muscle is thicker around the bladder-urethral junction, forming the involuntary internal urethral sphincter. This sphincter remains closed unless one is voiding. At the distal end of the urethra is the external urethral sphincter made up of skeletal muscle which is under voluntary control.
Micturition
Micturition is the process of passing urine (urination or voiding).
When the bladder is full the detrusor muscle contracts, and the internal and external urethral sphincters open, thus allowing the passage of urine. Both the detrusor muscle and the internal urethral sphincter are composed of smooth muscle and are innervated by the autonomic nervous system.
Micturition is partially controlled by a spinal micturition reflex. As the bladder fills to about 200 ml stretch receptors transmit signals to the spinal cord and parasympathetic efferent nerve fibres lead to the bladder contracting and the internal urethral sphincter opening. Involuntary voiding would occur if it were not for higher brain centres in the pons that also receive signals from stretch receptors.
If it is appropriate the pons sends signals to the spinal interneurons to contract the detrusor muscle, open the internal urethral sphincter and urine is voided.
If not appropriate, then signals from the cerebrum excite spinal interneurons that keep the internal urethral sphincter closed. Once it is timely to void, then these interneurons are inhibited and the external urethral sphincter, which is under voluntary control, relaxes and urine can be passed.
When the bladder is full the detrusor muscle contracts, and the internal and external urethral sphincters open, thus allowing the passage of urine. Both the detrusor muscle and the internal urethral sphincter are composed of smooth muscle and are innervated by the autonomic nervous system.
Micturition is partially controlled by a spinal micturition reflex. As the bladder fills to about 200 ml stretch receptors transmit signals to the spinal cord and parasympathetic efferent nerve fibres lead to the bladder contracting and the internal urethral sphincter opening. Involuntary voiding would occur if it were not for higher brain centres in the pons that also receive signals from stretch receptors.
If it is appropriate the pons sends signals to the spinal interneurons to contract the detrusor muscle, open the internal urethral sphincter and urine is voided.
If not appropriate, then signals from the cerebrum excite spinal interneurons that keep the internal urethral sphincter closed. Once it is timely to void, then these interneurons are inhibited and the external urethral sphincter, which is under voluntary control, relaxes and urine can be passed.